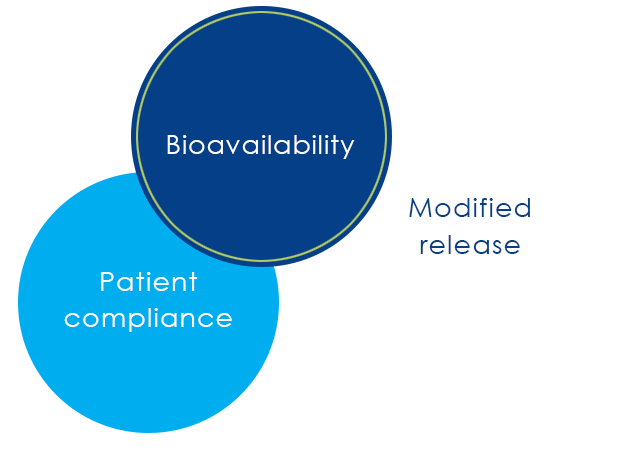
Bioverfügbarkeit
Lösungen zur Bioverfügbarkeit
How Losan Pharma Solves Bioavailability Challenges
What We Do
Using different platform technologies over the years, Losan has acquired in-depth experience on how to successfully improve oral bioavailability:
Nanotechnologie
One of the most suitable technologies for improving the solubility of poorly soluble compounds is to reduce the particle size to the nano size range, which increases the effective surface area. This results in much higher dissolution rates and an oversaturation effect that leads to higher bioavailability and lower required dosages. It also helps to reduce any food or adverse effects.
Our nanotechnology
Heißschmelzextrusion
Another approach is to use HME to form a solid drug dispersion with hydrophilic polymers. The drug is molecularly dispersed and has a lower thermodynamic barrier for dissolution. Solid dispersions are an effective formulation technique to improve solubility, dissolution rate and therefore the bioavailability of water-insoluble drugs for oral delivery. In addition, amorphous solid dispersion (ASD) can accelerate a project by offering flexible dose escalation and excipient acceptance for toxicology studies, as well as providing adequate preclinical and clinical exposure.
Our hot melt extrusion
Mesoporöse Silica Materialien
Another new and interesting approach in Losan’s portfolio of solid dispersions is silica amorphous loading (drug loading onto inert porous silica materials). Two processes are possible: a dry extrusion/milling step, or a solvent based set-up, where API particles are removed from an organic solution and loaded onto the pores providing large surface areas for drug adsorption. With this technology, amorphous drug loading up to 40% is possible and contributes to improving the bioavailability of poorly water-soluble drugs.
Our mesoporous silica technology
Sprühtrocknung
Spray drying is a well-established manufacturing technique which can be used to formulate amorphous solid dispersions by fast solvent evaporation that leads to a rapid transformation of solution to a solid state.
Besides evaporation kinetics, various process parameters that influence physical and chemical characteristics are important to obtain a stable amorphous structure of the API.
Our spray drying technology
Technologien der Bioverfügbarkeit
Losan Pharma Innovation: Bioverfügbarkeit | gesteuerte Wirkstofffreisetzung | Patienten Compliance