What experience does Losan have with contract analysis?
For more than 30 years we have been supporting our existing customers both with routine but also method development, troubleshooting, validation and stability analytics. This service is available from 2021 for every customer.
Does Losan work with a chromatography data system (CDS)?
We have a very broad based CDS (Chromeleon 7.2, Thermo Fisher) which guarantees you fast, standardised sequences and therefore minimal error and fast turnaround times.
Does Losan work with a Lab Information Management System (LIMS)?
Losan works with LabWare 8, the LIMS from LabWare®. With it, we electronically record and manage the samples to be analysed, the analyses performed and the results. Analysis certificates for the corresponding samples are generated directly via the LIMS. Stability studies, including individual results and stability reports, are also managed via the LIMS. As a result, we have modernised our workflows, moving away from paper-based documentation to rapid digital capture and data availability, and reducing the potential for errors.
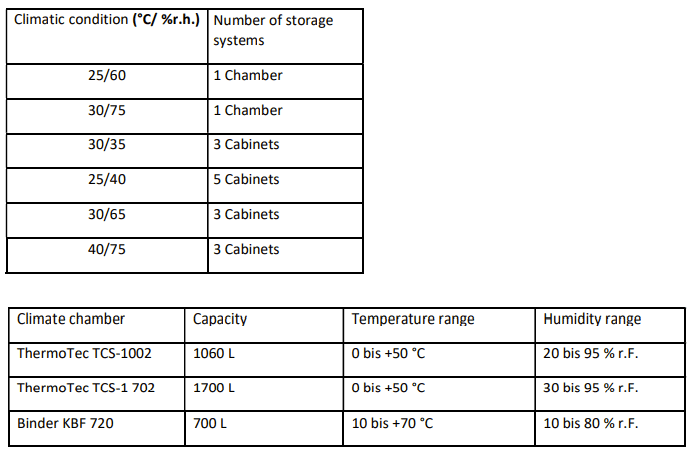
What stabilisation systems does Losan have and what climate conditions are offered?
Stability cabinets can be set to dedicated selectable conditions for temperature and humidity.
Which samples can be analysed?
APIs, intermediates, starting materials and finished drugs. Also highly potent substances up to OEL 3b. Narcotics.
Does Losan perform analytical method development in order to ensure that the analytical method is “stability indicating”?
We perform forced degradation studies on the active ingredient and finished product to develop a stability indicating method. Samples are stressed under the following conditions:
- Acid/ Base
- oxidation
- dry heat, moist heat
- light stress (ICHQ1B)
Does Losan perform stress testing according to ICHQ1B?
Losan uses Atlas’ suntest XLS + to perform photostability studies on APIs and drug products as per ICHQ1B.
Which guidelines does Losan use to perform method validation?
We work in accordance with ICH guidelines (ICH Q2R), with established pharmacopoeias (Ph.Eur, USP, BP, CAB, etc.) as well as with dedicated customer specifications and requests.
What do reports/ CoAs look like?
We generate the respective analysis reports and document them in Losan’s standard layout. Customers appreciate the clear structure, uniformity and clarity of our reports. Naturally, we can also work with individual customer templates or according to customer requirements.
Based on our customers’ respective specifications and requirements, we prepare Certificates of Analysis (CoA) using the unified Losan standard.
Which analytical lab equipments / instruments are used?
We adapt our modern, state-of-the-art instrumentation to changing regulations and the ongoing needs of our clients. Discover our portfolio and the analytical equipment we use.
Losan Pharma: Our company | Quality | Innovation