Hot Melt Extrusion
Hot melt extrusion
The percentage of drug candidates with relatively poor biopharmaceutical properties, and particularly candidates with poor dissolution properties, has increased significantly over the past 10 years. These substances require considerable formulation effort to achieve their dosage forms.
HME offers a possible solution and has been established for many years.
Well-known products such as Novir® and Keltra® are commercially available.
Losan Pharma successfully implemented HME 10 years ago and has continued to develop it ever since. We are one of the world’s leading suppliers (CMDO) of this technology.
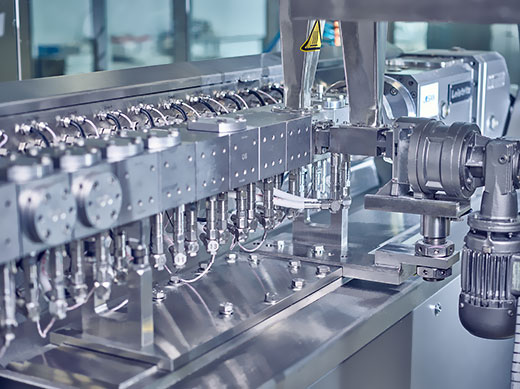


HME complements other drug delivery technologies such as nanotechnology, amorphous loading of mesoporous silicate carriers or liposomal encapsulation. The specific properties of each drug and its target profile (TPP) determine which of these technologies is most appropriate.
We help our customers discover many advantages of HME technology

Cost reduction
Losan has sophisticated screening methods to determine potential polymer loading, solubility enhancement, permeability, and formulation stability. Also in this area, we are constantly trying to improve and are collaborating with university institutions to further optimize the predictive probability of our models. Hence, in a short period of time, formulations on a milligram scale allow a reliable formulation selection. This saves costs and valuable time during development.
Enhancing solubility and bioavailability
HME enables the amorphous encapsulation of active ingredients in water-soluble carriers. In addition to taste masking, such as for paediatric applications, a significant improvement in the solubility and bioavailability of poorly soluble active ingredients can be achieved.
Our HME technology
We offer the following services:
Hot melt extrusion at Losan Pharma
Suitable and stable formulation development based on the respective target profile and the available physicochemical properties of the active ingredient
Stability data determination (ASAP or short-term stability data at different ICH conditions)
Analytical characterisation of formulations e.g., with respect to crystallinity/amorphous encapsulation (powder diffraction and electron microscopy), solubility and chemical stability
Supply of prototype formulations for animal studies directly from screening
Support with the design and performance of exploratory pK studies in animals
Main advantages of HME
HME’s main advantages can be summarized as follows:
No use of organic solvents, such as those used in spray drying
Use of GRAS listed, easily tolerated excipients in small quantities to stabilize the amorphously encapsulated active ingredients
Easy and targeted scaling up from screening to production scale
Excellent batch reproducibility due to intensive mixing process
Significant increase in bioavailability and absorption of the encapsulated active ingredient
High active ingredient concentrations (up to 50%, w/w) are possible in the respective HME formulation depending on the particular active ingredient
Continuous production including cooling e.g., drum cooler and granule strand size reduction (hammer mill, granulator) to particles with targeted distribution
Our HME granules can be further processed, for example into tablets or mini tablets, capsules and granules.
Additionally, coating processes are available, for example for drug retardation or for targeted release in specific intestinal segments.
Filling HME granules, pellets or mini tablets in stick packs is also possible.
FAQs
For more information on HME and answers to frequently asked questions, please click here
We offer manufacturing and packaging of clinical trial samples for oral dosage forms under cGMP for active ingredients up to an OEL class of 3b (1-10 µg/m3).
Losan Pharma technologies: Hot melt extrusion | Hot melt coating | nanotechnology | mesoporous silica | stick packs | spray drying | coatings | effervescent tablets | matrix tablets | ODTs